
Actas del Congreso Nacional de
Tecnología Aplicada a Ciencias
de la Salud



Actas del Congreso Nacional de Tecnología Aplicada a Ciencias de la Salud Vol. 2, 2019
En este trabajo analizamos tres tipos de antraciclinas (ACys). El análisis consiste en observar cómo estos ACys interactúan con los neurotransmisores (NT). Estos medicamentos se usan en el tratamiento de diferentes tipos de cáncer en pacientes humanos. El programa Hyperchem se utilizó para realizar la simulación y los cálculos de variables cuánticas. Como resultado, los investigadores observamos que ACys tiene una alta probabilidad de interacción con NT. Esta interacción tiene diferentes efectos posibles dependiendo del compuesto que se analiza. Llegamos a la conclusión de que la parte del sistema nervioso central (SNC) que controla el estado de ánimo del paciente se ve afectada.
Palabra clave. Antraciclinas, neurotransmisores, Hyperchem, análisis cuántico
In this work we analyze three types of anthracyclines (ACys). The analysis consists of observing how these ACys interact with the neurotransmitters (NT). These drugs are used in the treatment of different types of cancer in human patients. The Hyperchem program was used to perform the simulation and calculations of quantum variables. As a result, we researchers observed that ACys have a high probability of interaction with NT. This interaction has different possible effects depending on the compound being analyzed. We conclude that the part of the central nervous system (CNS) that controls the mood of the patient is affected.
Keyword. Anthracyclines, Neurotransmitters, Hyperchem, Quantum Analysis
Clinical use of the three ACys
Daunorubicin is a chemotherapeutic drug of the ACy family that is used to treat certain types of cancer. Some specific types of leukemia, such as acute myeloid leukemia and acute lymphoid leukemia [4].
Epirubicin is a medication that belongs to the ACy family and is used as chemotherapy to treat cancer. It causes fewer side effects due to its rapid elimination. It is primarily used in the treatment of breast cancer, ovarian cancer, stomach cancer, lung cancer and lymphomas [5-8].
Doxorubicin is a drug that belongs to the ACy family and is used as chemotherapy to treat cancer. Doxorubicin is commonly used in the treatment of some leukemia and Hodgkin's lymphoma, as well as cancer of the bladder, breast, stomach, lung, ovaries, thyroid, multiple myeloma and others [9-11].
Currently, there are no known studies that predict the effects of ACys on NT or AA. On the other hand, ACys attack DNA synthesis and replication, but very little is known about the active site of the attack to break the chain [12-13].
In the other hands, NTs are chemicals created by the body that transmit information signals from a neuron through contact points called synapses. The knowledge about neurotransmitters is fundamental to understand the human being. It is linked to the nervous system and the adaptation of the mind to each process [14-16].
Therefore, the article focuses on the quantum analysis of three basic types of ACys used for different types of cancer and the possible chemical interaction of compounds with the main neurotransmitters [17-20].
We perform an analysis of the ACys to know how they affect human body functions.
For this, the HYPERCHEM quantum interaction program is used, which is a quantum analysis tool to compare compounds in pairs using their chemical characteristics [21-24].
This article was evaluated by researchers for three different types of ACys most commonly used as drugs against different types of cancer. The neurotransmitters that we choose for this analysis are the most representative.
It selected specific parameters for each of the simulations shows in Table 1 and 2[26].
Table 1. Parameters used for quantum computing molecular orbitals HOMO and LUMO and BG
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Total Charge | 0 | Polarizability | Not |
| Spin Multiplicity | 1 | Geometry Optimization: Algoritm | Polak-Ribiere (Conjugated gradient) |
| Spin Pairing | RHF | Termination condition RMS gradient of | 0.1 kcal/Amol |
| State Lowest Converget Limit | 0.01 | Termination condition or | 195 maximum cycles |
| Interation Limit | 50 | Termination condition or | In vacuo |
| Accelerate Convergence | Yes | Screen refresh period | 1 cyclest |
Table 2. Parameters used for quantum computing E-, E+ and EP
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Molecular Property | Property Electrostatic Potential | Contour Grid Increment | 0.05 |
| Representation | 3D Mapped Isosurface | Mapped Function Options | Default |
| Isosurface Grid: Grid Mesh Size | Coarse | Transparency level | A criteria |
| Isosurface Grid: Grid Layaout | Default | Isosurface Rendering: Total charge density contour value | 0.015 |
| Contour Grid: Starting Value | Default | Rendering Wire Mesh | Default |
Table 3 shows the interactions of all the pure substances selected for this study. It can be seen that DAUNORUBICIN is the most chemically stable drug; while ACETYLCHOLINE is the most unstable NT of all of them.
In ascending order, greater stability is noted in the EPIRUBICIN than in the DOXORUBICIN. However, ADRENALINE is interspersed among the three drugs. For this reason, it is assumed that ADRENALINE can be attacked by them very quickly.
Table 3. ETCs of pure substances. The DAUNORUBICIN is the most stable of all. The ACETYLCHOLINE is the most unstable of all
| SUBSTANCE | HOMO | LUMO | BG | E- | E+ | EP | ETC |
|---|---|---|---|---|---|---|---|
| ACETYLCHOLINE | -9.242 | 1.034 | 10.276 | -0.028 | 0.105 | 0.133 | 77.265 |
| NORADRENALINE | -9.152 | -0.004 | 9.148 | -0.083 | -0.222 | 0.139 | 65.810 |
| GLUTAMIC ACID | -10.044 | 0.537 | 10.582 | -0.084 | 0.197 | 0.281 | 37.657 |
| ASPARTIC ACID | -10.242 | 0.516 | 10.758 | -0.109 | 0.198 | 0.307 | 35.042 |
| GLYCINE | -9.853 | 0.874 | 10.727 | -0.126 | 0.188 | 0.314 | 34.164 |
| GABA | -9.562 | 0.939 | 10.500 | -0.140 | 0.180 | 0.320 | 32.813 |
| DOPAMINE | -8.868 | 0.199 | 9.067 | -0.098 | 0.189 | 0.287 | 31.591 |
| SEROTONINE | -8.948 | -0.129 | 8.819 | -0.145 | 0.141 | 0.286 | 30.836 |
| DOXORUBICIN* | -9.299 | -0.605 | 8.694 | -0.115 | 0.186 | 0.301 | 28.885 |
| ADRENALIN | -8.998 | 0.092 | 9.090 | -0.117 | 0.198 | 0.315 | 28.858 |
| EPIRUBICIN* | -8.932 | -1.235 | 7.696 | -0.109 | 0.187 | 0.296 | 26.000 |
| DAUNORUBICIN* | -8.955 | -1.293 | 7.662 | -0.137 | 0.188 | 0.325 | 23.575 |
*The drugs that interact with NTs.
In table 4 we can see the molecule-to-molecule interactions of drugs and NTs. It is important to note that the interactions of the three drugs show a similar pattern. It can be seen in this table that the medicines oxidize the NT with a very high probability. However, NTs have a very low likelihood of oxidizing all three drugs.
We designed, figures 1, 2, 3, and four were to clarify with more precision this event; They illustrate a similar pattern where each NT gives electrons to drugs. Serotonin is the most affected by drugs, then followed by GABA, ADRENALINE, DOPAMINE, GLYCINE.
On the other hand, some interactions between drugs are observed. These undesired interactions may cause a potential deficiency or an increase in the potential of the drugs for their therapeutic effect or their unwanted side reactions (interactions: 60, 54, 53, 49 table 4)
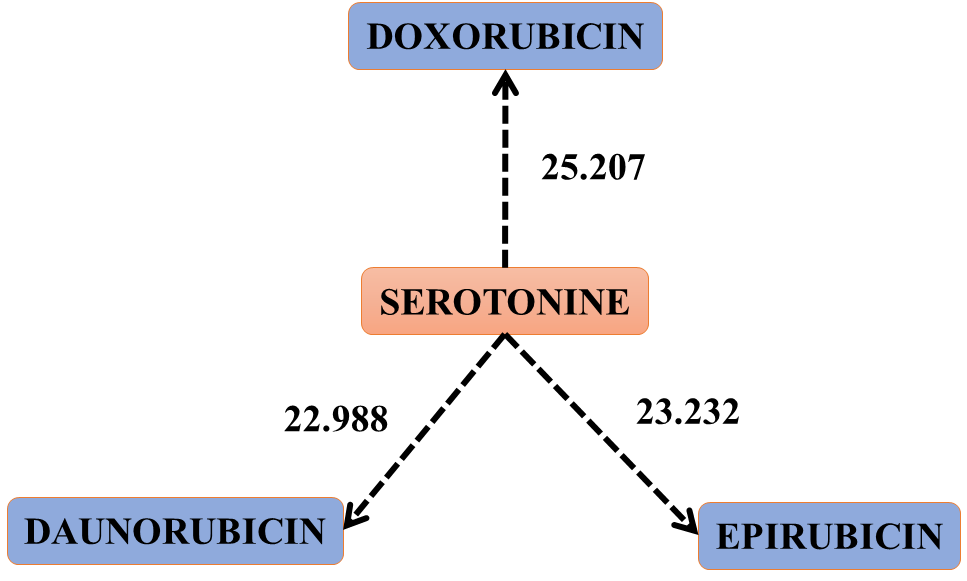
Figure 1. The scheme that shows the jump of electrons or electronic clouds from serotonin to the three drugs.Interactions: 59, 62 and 63 of table 4. This figure represents the oxidation of SEROTONINE by the three drugs
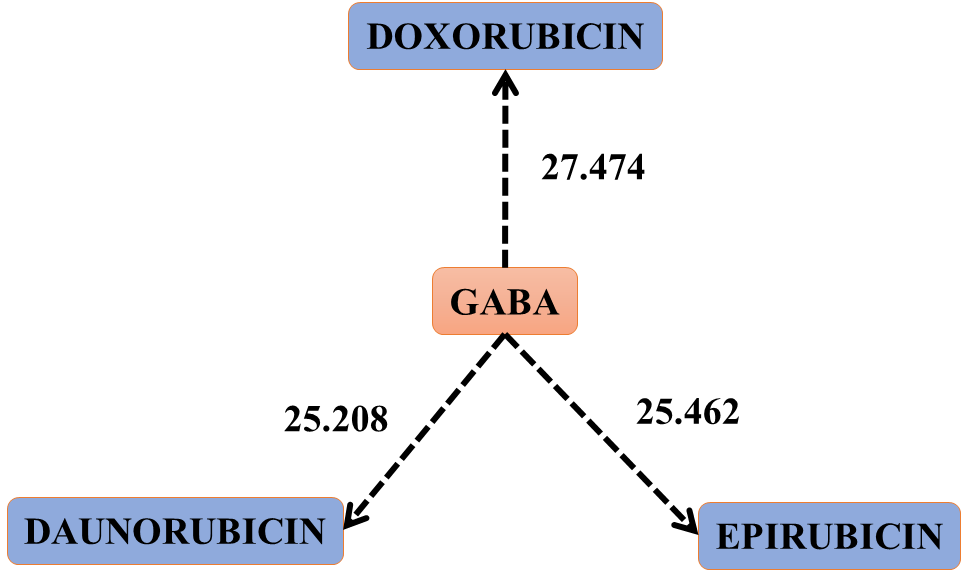
Figure 2. Scheme that shows the jump of electrons or electronic clouds from GABA to the three drugs. Interactions:58, 56 and 45 of table 4. This figure represents the oxidation of GABA by the three drugs
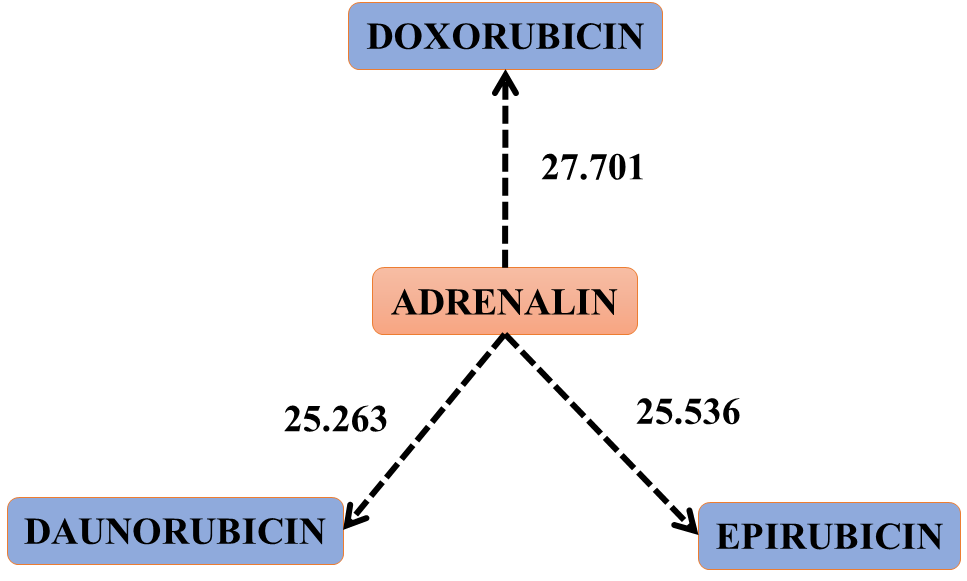
Figure 3. Scheme that shows the jump of electrons or electronic clouds from ADRENALIN to the three drugs. Interactions: 57, 55 and 43 of table 4. This figure represents the oxidation of ADRENALIN by the three drugs
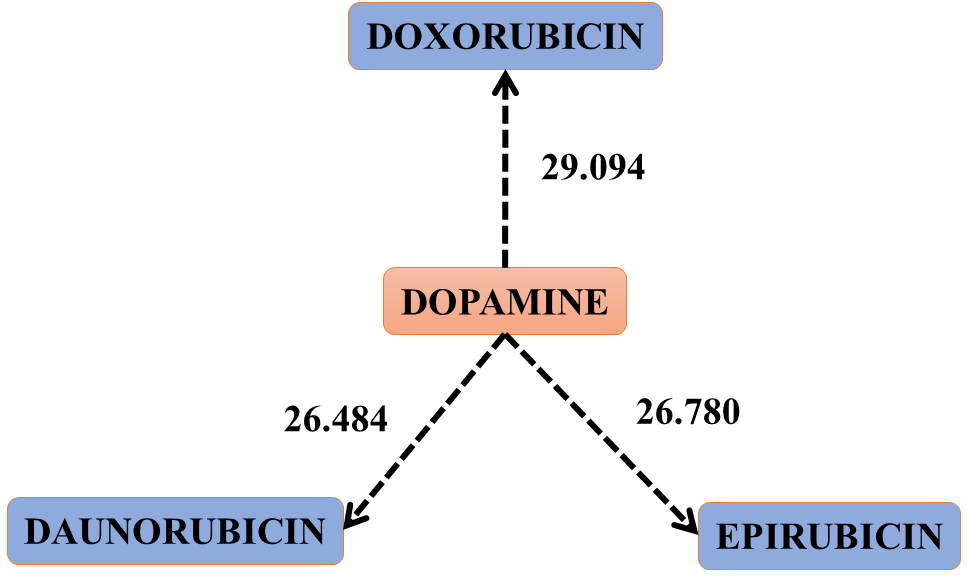
Figure 4. Scheme that shows the jump of electrons or electronic clouds from DOPAMINE to the three drugs. Interactions: 50, 48 and 36 of table 4. This figure represents the oxidation of DOPAMINE by the three drugs
SEROTONIN is known as a very important NT and is related to the state of mind that a person can adopt. There may be a depressive effect in patients who undergo treatment with the 3 drugs. Similarly, ADRENALIN is one of the best known NT and is responsible for increasing the metabolism and alerting it if there is any danger. When interacting with this tree, it could cause a deficit of energy in the patients that would lead to low levels of metabolism in the body. On the other hand, GABA affects the nervous system, which corroborates the above.
Table 4. The ETCs or crossed bands. It is observed that the NT most oxidized by th drug DAUNORUBICIN is the serotonin
| SUBSTANCE | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | REDUCING AGENT | OXIDIZING AGENT | HOMO | LUMO | BG | E- | E+ | EP | ETC |
| 1 | DAUNORUBICIN | NORADRENALINE | -8.955 | -0.004 | 8.951 | -0.137 | -0.222 | 0.085 | 105.303 |
| 2 | DOXORUBICIN | NORADRENALINE | -9.299 | -0.004 | 9.295 | -0.115 | -0.222 | 0.107 | 86.870 |
| 3 | EPIRUBICIN | NORADRENALINE | -8.932 | -0.004 | 8.927 | -0.109 | -0.222 | 0.113 | 79.003 |
| 4 | DOXORUBICIN | ACETYLCHOLINE | -9.299 | 1.034 | 10.334 | -0.115 | 0.105 | 0.220 | 46.971 |
| 5 | EPIRUBICIN | ACETYLCHOLINE | -8.932 | 1.034 | 9.966 | -0.109 | 0.105 | 0.214 | 46.569 |
| 6 | DAUNORUBICIN | ACETYLCHOLINE | -8.955 | 1.034 | 9.989 | -0.137 | 0.105 | 0.242 | 41.278 |
| 7 | ACETYLCHOLINE | DOXORUBICIN | -9.242 | -0.605 | 8.637 | -0.028 | 0.186 | 0.214 | 40.360 |
| 8 | ACETYLCHOLINE | EPIRUBICIN | -9.242 | -1.235 | 8.007 | -0.028 | 0.187 | 0.215 | 37.240 |
| 9 | ACETYLCHOLINE | DAUNORUBICIN | -9.242 | -1.293 | 7.949 | -0.028 | 0.188 | 0.216 | 36.800 |
| 10 | DOXORUBICIN | SEROTONINE | -9.299 | -0.129 | 9.170 | -0.115 | 0.141 | 0.256 | 35.820 |
| 11 | EPIRUBICIN | SEROTONINE | -8.932 | -0.129 | 8.802 | -0.109 | 0.141 | 0.250 | 35.209 |
| 12 | GLUTAMIC ACID | DOXORUBICIN | -10.044 | -0.605 | 9.439 | -0.084 | 0.186 | 0.270 | 34.961 |
| 13 | DOXORUBICIN | GABA | -9.299 | 0.939 | 10.238 | -0.115 | 0.180 | 0.295 | 34.705 |
| 14 | EPIRUBICIN | GABA | -8.932 | 0.939 | 9.870 | -0.109 | 0.180 | 0.289 | 34.153 |
| 15 | DOXORUBICIN | GLYCINE | -9.299 | 0.874 | 10.174 | -0.115 | 0.188 | 0.303 | 33.577 |
| 16 | EPIRUBICIN | GLYCINE | -8.932 | 0.874 | 9.806 | -0.109 | 0.188 | 0.297 | 33.017 |
| 17 | ASPARTIC ACID | DOXORUBICIN | -10.242 | -0.605 | 9.637 | -0.109 | 0.186 | 0.295 | 32.667 |
| 18 | GLUTAMIC ACID | EPIRUBICIN | -10.044 | -1.235 | 8.809 | -0.084 | 0.187 | 0.271 | 32.505 |
| 19 | GLUTAMIC ACID | DAUNORUBICIN | -10.044 | -1.293 | 8.751 | -0.084 | 0.188 | 0.272 | 32.173 |
| 20 | NORAADRENALINE | DOXORUBICIN | -9.152 | -0.605 | 8.547 | -0.083 | 0.186 | 0.269 | 31.772 |
| 21 | DAUNORUBICIN | SEROTONINE | -8.955 | -0.129 | 8.826 | -0.137 | 0.141 | 0.278 | 31.747 |
| 22 | DOXORUBICIN | GLUTAMIC ACID | -9.299 | 0.537 | 9.836 | -0.115 | 0.197 | 0.312 | 31.527 |
| 23 | DOXORUBICIN | ASPARTIC ACID | -9.299 | 0.516 | 9.816 | -0.115 | 0.198 | 0.313 | 31.359 |
| 24 | DOXORUBICIN | DOPAMINA | -9.299 | 0.199 | 9.498 | -0.115 | 0.189 | 0.304 | 31.244 |
| 25 | DAUNORUBICIN | GABA | -8.955 | 0.939 | 9.894 | -0.137 | 0.180 | 0.317 | 31.210 |
| 26 | EPIRUBICIN | GLUTAMIC ACID | -8.932 | 0.537 | 9.469 | -0.109 | 0.197 | 0.306 | 30.943 |
| 27 | EPIRUBICIN | ASPARTIC ACID | -8.932 | 0.516 | 9.448 | -0.109 | 0.198 | 0.307 | 30.774 |
| 28 | EPIRUBICIN | DOPAMINA | -8.932 | 0.199 | 9.130 | -0.109 | 0.189 | 0.298 | 30.639 |
| 29 | ASPARTIC ACID | EPIRUBICIN | -10.242 | -1.235 | 9.006 | -0.109 | 0.187 | 0.296 | 30.427 |
| 30 | DAUNORUBICIN | GLYCINE | -8.955 | 0.874 | 9.829 | -0.137 | 0.188 | 0.325 | 30.245 |
| 31 | ASPARTIC ACID | DAUNORUBICIN | -10.242 | -1.293 | 8.949 | -0.109 | 0.188 | 0.297 | 30.130 |
| 32 | DOXORUBICIN | ADRENALIN | -9.299 | 0.092 | 9.391 | -0.115 | 0.198 | 0.313 | 30.003 |
| 33 | GLYCINE | DOXORUBICIN | -9.853 | -0.605 | 9.248 | -0.126 | 0.186 | 0.312 | 29.641 |
| 34 | EPIRUBICIN | ADRENALIN | -8.932 | 0.092 | 9.023 | -0.109 | 0.198 | 0.307 | 29.392 |
| 35 | NORAADRENALINE | EPIRUBICIN | -9.152 | -1.235 | 7.916 | -0.083 | 0.187 | 0.270 | 29.320 |
| 36 | DOPAMINE | DOXORUBICIN | -8.868 | -0.605 | 8.263 | -0.098 | 0.186 | 0.284 | 29.094 |
| 37 | NORAADRENALINE | DAUNORUBICIN | -9.152 | -1.293 | 7.859 | -0.083 | 0.188 | 0.271 | 28.998 |
| 38** | DOXORUBICIN | DOXORUBICIN | -9.299 | -0.605 | 8.694 | -0.115 | 0.186 | 0.301 | 28.885 |
| 39 | DAUNORUBICIN | GLUTAMIC ACID | -8.955 | 0.537 | 9.492 | -0.137 | 0.197 | 0.334 | 28.420 |
| 40 | DAUNORUBICIN | ASPARTIC ACID | -8.955 | 0.516 | 9.471 | -0.137 | 0.198 | 0.335 | 28.272 |
| 41 | EPIRUBICIN | DOXORUBICIN | -8.932 | -0.605 | 8.327 | -0.109 | 0.186 | 0.295 | 28.226 |
| 42 | DAUNORUBICIN | DOPAMINA | -8.955 | 0.199 | 9.154 | -0.137 | 0.189 | 0.326 | 28.079 |
| 43 | ADRENALIN | DOXORUBICIN | -8.998 | -0.605 | 8.393 | -0.117 | 0.186 | 0.303 | 27.701 |
| 44 | GLYCINE | EPIRUBICIN | -9.853 | -1.235 | 8.618 | -0.126 | 0.187 | 0.313 | 27.532 |
| 45 | GABA | DOXORUBICIN | -9.562 | -0.605 | 8.957 | -0.140 | 0.186 | 0.326 | 27.474 |
| 46 | GLYCINE | DAUNORUBICIN | -9.853 | -1.293 | 8.560 | -0.126 | 0.188 | 0.314 | 27.260 |
| 47 | DAUNORUBICIN | ADRENALIN | -8.955 | 0.092 | 9.047 | -0.137 | 0.198 | 0.335 | 27.005 |
| 48 | DOPAMINE | EPIRUBICIN | -8.868 | -1.235 | 7.632 | -0.098 | 0.187 | 0.285 | 26.780 |
| 49 | DOXORUBICIN | EPIRUBICIN | -9.299 | -1.235 | 8.064 | -0.115 | 0.187 | 0.302 | 26.702 |
| 50 | DOPAMINE | DAUNORUBICIN | -8.868 | -1.293 | 7.575 | -0.098 | 0.188 | 0.286 | 26.484 |
| 51 | DOXORUBICIN | DAUNORUBICIN | -9.299 | -1.293 | 8.006 | -0.115 | 0.188 | 0.303 | 26.423 |
| 52** | EPIRUBICIN | EPIRUBICIN | -8.932 | -1.235 | 7.696 | -0.109 | 0.187 | 0.296 | 26.000 |
| 53 | DAUNORUBICIN | DOXORUBICIN | -8.955 | -0.605 | 8.350 | -0.137 | 0.186 | 0.323 | 25.851 |
| 54 | EPIRUBICIN | DAUNORUBICIN | -8.932 | -1.293 | 7.638 | -0.109 | 0.188 | 0.297 | 25.718 |
| 55 | ADRENALIN | EPIRUBICIN | -8.998 | -1.235 | 7.763 | -0.117 | 0.187 | 0.304 | 25.536 |
| 56 | GABA | EPIRUBICIN | -9.562 | -1.235 | 8.326 | -0.140 | 0.187 | 0.327 | 25.462 |
| 57 | ADRENALIN | DAUNORUBICIN | -8.998 | -1.293 | 7.705 | -0.117 | 0.188 | 0.305 | 25.263 |
| 58 | GABA | DAUNORUBICIN | -9.562 | -1.293 | 8.268 | -0.140 | 0.188 | 0.328 | 25.208 |
| 59 | SEROTONINE | DOXORUBICIN | -8.948 | -0.605 | 8.343 | -0.145 | 0.186 | 0.331 | 25.207 |
| 60 | DAUNORUBICIN | EPIRUBICIN | -8.955 | -1.235 | 7.720 | -0.137 | 0.187 | 0.324 | 23.826 |
| 61** | DAUNORUBICIN | DAUNORUBICIN | -8.955 | -1.293 | 7.662 | -0.137 | 0.188 | 0.325 | 23.575 |
| 62 | SEROTONINE | EPIRUBICIN | -8.948 | -1.235 | 7.713 | -0.145 | 0.187 | 0.332 | 23.232 |
| 63 | SEROTONINE | DAUNORUBICIN | -8.948 | -1.293 | 7.655 | -0.145 | 0.188 | 0.333 | 22.988 |
** The drugs that interact with NTs. These interactions mark the limits for electron transfer. All the interactions below them have a high probability that they can be carried out.
We studied the interactions between NTs and three ACys.
We found that the interactions NTs vs. ACys have a very similar pattern in all of them.
The ACys always oxidize the NTs.
We found that SEROTONINE is the most affected of the NTs that were studied.
Our findings coincide with the medical literature of the side effects of these three drugs when used to treat any disease.